Researchers from Flinders University in South Australia and Zhejiang Sci-Tech University in China have developed the world’s first aqueous aluminum radical battery. The new battery uses water-based electrolytes instead of hazardous materials, making it safe, efficient, and non-toxic. The battery can deliver a stable voltage output of 1.25 V and a capacity of 110 mAh g–1 over 800 cycles with minimal degradation.
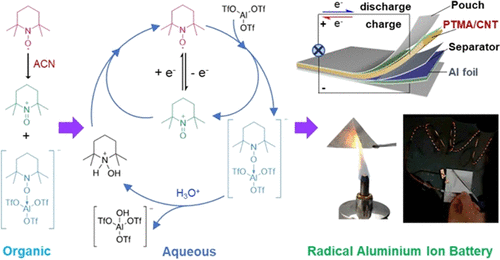
The team began their investigations by exploring the electrochemistry of stable radicals – focussing on the most commonly utilized Lewis acid electrolyte – Al(Otf)3 – along with battery testing. Though widely used in different organic battery systems, stable radicals have never been applied in aluminum-ion batteries (AIBs) before, due to a lack of understanding of their electrochemical reaction in electrolytes. They discovered that a stable radical – called TEMPO – can undergo reversible disproportionation in the presence of a Lewis acid, which enables the formation of aluminum radical batteries.
Professor Zhongfan Jia, from Flinders University’s College of Science and Engineering, explained that aluminum-ion batteries have great potential as an alternative to lithium-ion batteries as they use abundant elements of the Earth’s crust and provide a much higher energy density than lithium-ion batteries. However, one of the major challenges for current AIBs was the slow movement of Al3+ ion complexes, which led to AIBs with low cathode efficiency. Jia states that their novel design of aluminum radical batteries solves this problem by using stable radicals as cathodes, which could achieve a high voltage output and a high capacity with excellent cycling stability.
Not only does the new battery use water-based electrolytes that are fire-retardant and air-stable, it also uses biodegradable materials for the soft-pack batteries to reduce the environmental impact. The researchers plan to investigate the further optimization of the battery performance and scale up the production for commercialization.
