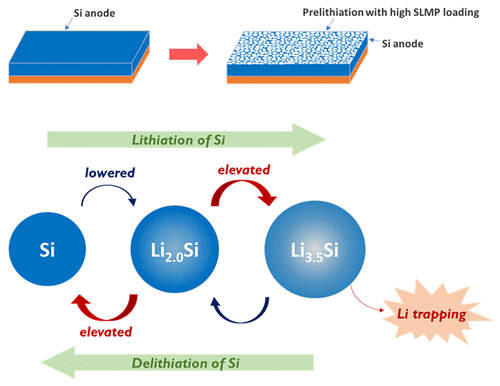
A team from Rice University’s George R. Brown School of Engineering has developed a readily scalable, new priming method that improves battery life by up to 44%. The method optimizes prelithiation, which introduces extra active lithium ions into the battery so that the lithium loss during the first charge and long-term cycling can be compensated, thus raising the energy density and elongating the battery’s life cycle life.
The team – led by Dr. Sibani Lisa Biswal – spray-coated the battery’s anodes with a mixture of stabilized lithium metal particles (SLMPs) and a surfactant and was able to improve battery life by 22% to 44%. The battery cells with a greater amount of the coating initially achieved a higher stability and cycle life, however, when cycled at full capacity, a larger amount of the particle coating led to more lithium trapping, causing the battery to fade more rapidly in subsequent cycles. This was caused by the formation of a solid-electrolyte interphase – or SEI layer – which is formed when the electrolyte in a battery cell reacts with electrons and lithium ions, resulting in a nanometer-scale layer of salts deposited on the anode. This layer insulates the electrolyte from the anode, preventing the reaction from continuing.
Their new prelithiation method improves SEI layer stability, which means fewer lithium ions are depleted when it is formed.
“Prelithiation is a strategy designed to compensate for the lithium loss that typically occurs with silicon,” Biswal said. “You can think of it in terms of priming a surface, like when you’re painting a wall and you need to first apply an undercoat to make sure your paint sticks. Prelithiation allows us to ‘prime’ the anodes so batteries can have a much more stable, longer cycle life.”
Though not a new method, the team has been able to improve the process so that it can be readily incorporated into existing battery manufacturing processes.
“One aspect of the process that is definitely new and that Quan (Quan Anh Nguyen, lead author) developed was the use of a surfactant to help disperse the particles,” Biswal said. “This has not been reported before, and it’s what allows you to have an even dispersion. So instead of them clumping up or building up into different pockets within the battery, they can be uniformly distributed.”
